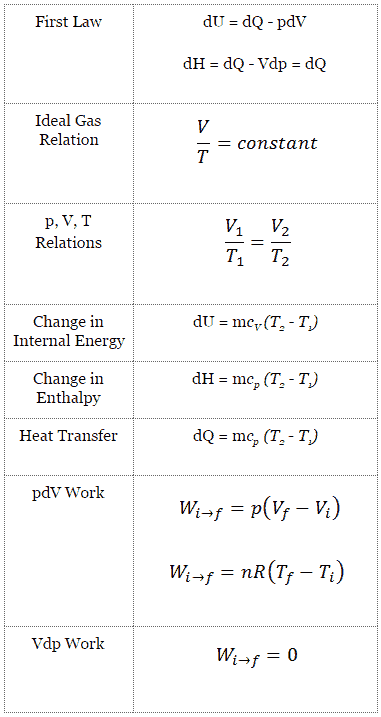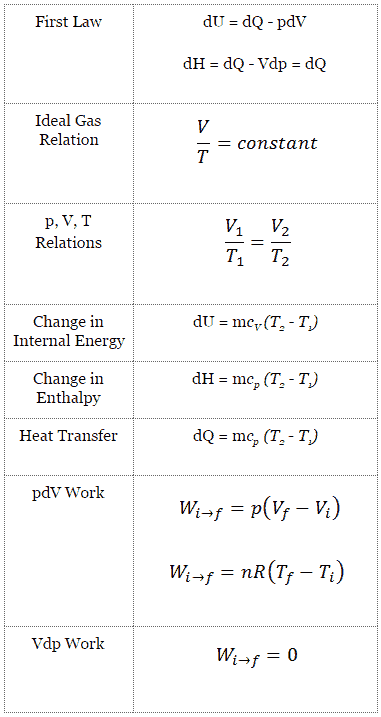It keeps account of the gains and losses of energy of the system that are due to changes in its internal state.
Change in specific internal energy formula.
So that gives us that delta u or change in internal energy is negative 485 joules then if we plug this all into our calculator to calculate the work we get positive 25 25 joules.
For a temperature change at constant volume dv 0 and by definition of heat capacity d q v c v dt.
If a certain amount of heat is applied to gas the result is that the temperature of the gas may increase or else the volume of gas might increase.
Internal energy u the third component of our closed system energy equation is the change of internal energy resulting from the transfer of heat or work.
The change in the internal energy of a system is the sum of the heat transferred and the work done.
Consider for example the following solved problem.
The internal energy is measured as a difference from a.
An ideal gas with specific heats independent of temperature and is referred to as a perfect gas for example monatomic gases and diatomic gases at ordinary temperatures are considered perfect gases.
Specific energy or massic energy is energy per unit mass it is also sometimes called gravimetric energy density or just energy density though energy density more precisely means energy per unit volume it is used to quantify for example stored heat and other thermodynamic properties of substances such as specific.
Internal energy formula is the heat energy stocked in gas.
Where and have been used to denote the specific heats for one kmol of gas and is the universal gas constant.
In this manner doing some work externally or volume and temperature may both intensify but it will be made definite by the situations.
To understand the relationship between work and heat we need to understand the factor of linking factors.
Since specific internal energy is a property of the system it is usually presented in the property tables such as in the steam tables.
With the interactions of heat work and internal energy there are energy transfers and conversions every time a change is made upon a system.
The specific heat ratio or is a function of only and is greater than unity.
We cannot create nor destroy energy but we can convert or transfer it.
So if we add our heat and our work here we get that the overall change in internal energy for this process is negative 460 joules.
The heat flow is equal to the change in the internal energy of the system plus the pv work done.
Internal energy refers to all the energy within a given system including the kinetic energy of molecules and the energy stored in all of the chemical bonds between molecules.
Energy density has tables of specific energies of devices and materials.
When the volume of a system is constant changes in its internal energy can be calculated by substituting the ideal gas law into the equation for δu.
This is the change in internal energy.